top of page
[2+2+2] Cycloadditions of Siloxy Alkynes with 1,2-Diazines: From Reaction Discovery to Identification of an Antiglycolytic Chemotype
Dr. Timothy J. Montavon, Dr. Yunus E. Türkmen, Noumaan A. Shamsi, Christopher Miller, Chintan S. Sumaria, Prof. Dr. Viresh H. Rawal, and Prof. Dr. Sergey A. Kozmin
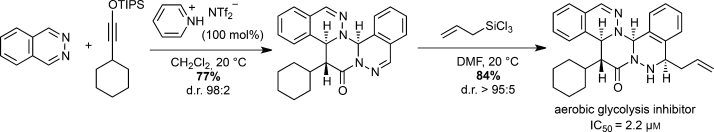
A newly uncovered Brønsted acid-promoted [2+2+2] cycloaddition between siloxy alkynes and 1,2-diazines produces novel polycyclic compounds with high efficiency and excellent diastereoselectivity under exceedingly mild conditions. A small-molecule library synthesized using this reaction yielded a novel chemotype, which inhibited glycolytic ATP production by blocking glucose uptake in CHO-K1 cells.
ANGEWANDTE CHEMIE 52:51 (16 Dec. 2013): 13576-79
bottom of page